Individuals age because their cells age. Although public attention on the aging process has traditionally focused on its cosmetic manifestations, aging has important health consequences
CELLULAR AGING
People age because their cells age. Although public attention to the aging process has traditionally focused on its aesthetic manifestations, aging is one of the most important independent risk factors for many chronic diseases, such as cancer, heart disease, Alzheimer's and ischemic heart disease. Aging therefore has a significant impact on health. mad Perhaps one of the most striking findings about cellular senescence is that cellular senescence is not simply the result of 'steam exhaustion' of cells, but is actually regulated by a limited number of evolutionarily conserved genes and signaling pathways in yeast. to mammals
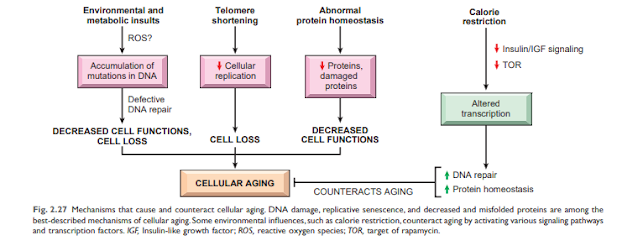
Cellular senescence is the result of a progressive reduction in the lifespan and functional activity of cells. Several abnormalities contribute to cellular senescence (Figure 2). 2.27):
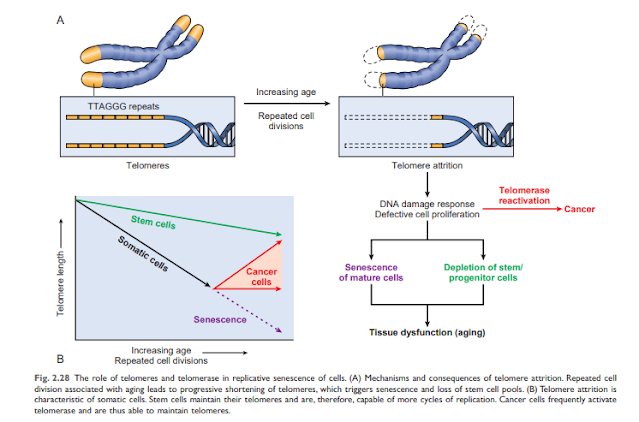
• Accumulation of mutations in DNA. A variety of metabolic insults can lead to DNA and mitochondrial damage over time. Exposure to radiation and oxygen from toxins contributes to the DNA damage associated with aging. While most DNA damage is repaired by DNA repair enzymes, some persist and accumulate as cells age, especially if repair mechanisms become ineffective over time. Accumulation of mutations in nuclear and mitochondrial DNA ultimately impairs functional activities and cell survival. • Reduction of cell proliferation. Normal cells (with the exception of stem cells) have a limited ability to reproduce and, after a certain number of divisions, stop in an indivisible state called proliferative aging. Aging is associated with progressive and recurrent aging of cells. Pediatric cells can proliferate more than older cells. In contrast, the cells of patients with Werner syndrome, a rare disease characterized by premature aging, shrink dramatically in the laboratory age. The aging resolved in aging cells occurs for Teloma's progressive default to arrest the final cell cycle. Teromamers are important to ensure full replication of chromosomes and shorten short series of DNA at the end of Teromostum are important to protect the end of solubility and deterioration. Teromer DNA also connects proteins that protect and intervene with activating DNA damage. When physical cells are copied, a small amount of teromer is not arranged twice, and pods are gradually shorter. Short and protect the end of chromosome, and can not be discovered as broken nuclear action to stop the cell cycle in cells. Telomere length is maintained by the addition of nucleotides by an enzyme called telomerase. Telomerase is a special set of RNA proteins that use RNA as a template for adding nucleotides to the ends of chromosomes. Telomerase is expressed in germ cells and is present at low levels in stem cells, but not in most somatic cells (Fig. 2.28). Therefore, as mature somatic cells age, their telomeres shorten and end the cell cycle, making them unable to generate new cells to replace damaged cells. Conversely, in immortalized cancer cells, telomere depletion is usually reactivated, telomere length stabilizes, and cells can grow indefinitely. This will be explained in detail in Chapter 6. Shortening telomeres can also reduce the ability of stem cells to regenerate and contribute to cell aging. Despite such fascinating observations, the relationship between telomerase activity and age-related telomere length has not yet been fully established. Abnormal telomera maintenance includes aplastic anemia and other cell depletion (possibly due to impaired hematopoietic stem cells), young gray hair, abnormal skin and nail pigmentation, and lung and liver fibrosis. It is related to illness. ..
These disorders sometimes consider tolerant proteins.
• Homostasis from defective proteins. Over time, cells can not maintain natural protein hometostasis, given the increase in trading rotation and reduce the synthesis due to reducing protein translation and defective conversion (increasing natural proteins) and constructive (which are false protisiat. Destruction). The score can be reduced in proteins within many malicious effects on cellular survival, repeat and functions. Pass counterfeit proteins simultaneously exercise the loss of job protein and can brought its cable.
• There is great interest in identifying biochemistry paths facing aging, not only because of its clear therapeutic potential. (Search for "Erics Youry"), but also because of the interpretation of these tracks can tell us about mechanisms that cause aging Calorie restriction has been shown to delay aging and extend lifespan in all species tested, from flies to mice. Calorie restriction is now thought to alter signaling pathways that affect aging (Figure 2.27). Among the biochemical changes associated with calorie restriction that have been reported to play a role in the aging process is decreased activation of insulin-like growth factor receptor signaling involving downstream networks of kinases and transcription factors. Reduced IGF-1 signaling slows cell growth and metabolism, reduces DNA replication errors, improves DNA repair, and improves protein homeostasis. Calorie restriction also improves immunity. All of these prevent aging.
• Persistent inflammation. As we age, the accumulation of damaged cells, lipids, and other endogenous substances can activate the inflammasome pathway (Chapter 5), causing persistent low levels of inflammation. Secondly, inflammation leads to the development of chronic diseases such as atherosclerosis and type 2 diabetes. Cytokines produced during inflammatory responses can themselves cause cellular changes that worsen aging, and chronic metabolic disorders can accelerate the aging process.
Clinical observations and epidemiological studies have shown that physical activity and, as already mentioned, caloric restriction slow down the aging process, while stress, possibly via increased production of glucocorticoids, accelerates aging. Unfortunately, the exact mechanisms underlying these effects have yet to be defined and, for now, we all remain vulnerable to the test of time.
It is understood that the various forms of cellular disturbances and adaptations described in this chapter cover a wide spectrum, ranging from reversible and irreversible forms of acute cellular damage, to changes in cell size, growth and function, to consequences largely unavoidable. signs of age. These many different changes are discussed in this book because all cases of organ damage, and ultimately all cases of clinical disease, are due to disturbances in cellular structure and function.
Book Refrence: Robbins (tenth edition)