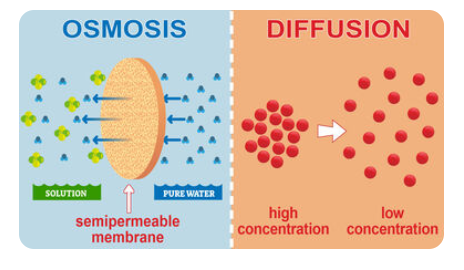
Diffusion is the continuous movement of molecules in a liquid or gas. Diffusion across cell membranes is divided into two subtypes:
Diffusion
• Simple diffusion means that molecules move through the membrane without binding to a carrier protein. Simple diffusion can occur in two ways.
(1) through the niche of the lipid bilayer
(2) via aqueous channels of transport proteins across the cell membrane.
• Facilitated diffusion requires carrier proteins. Carrier proteins aid the passage of molecules across the membrane, presumably by chemically binding to them and transporting them in this form across the membrane. The rate of diffusion of a substance across the cell membrane is directly proportional to its solubility in fats. The solubility of lipids for oxygen, nitrogen, carbon dioxide, anesthetic gases and most alcohols is so high that they can dissolve directly in the lipid bilayer and diffuse across the cell membrane.
Water and other insoluble lipid molecules diffuse through the protein channels in the cell membrane. Water easily penetrates the cell membrane and can also pass through the transmembrane protein channels. Other insoluble lipid molecules (mainly ions) can, like water molecules, pass through water-filled protein channels if they are small enough. Protein channels have selective permeability to transport one or more specific molecules. This permeability comes from the characteristics of the channel itself, such as its diameter, shape, and the nature of the charge flowing along its inner surface.
Synchronization of protein channels provides a method for controlling permeability. A gate is a gate-like extension of a carrier protein molecule, which can be closed over a channel opening or raised by changing the configuration in the protein molecule itself. The opening and closing of the gate is controlled in two main ways.
• Voltage blocking. In this case, the molecular stabilization of the gate responds to electrical potentials across the cell membrane. For example, the natural negative charge on the cell membrane causes the sodium gates to remain completely closed. When the inner space of the membrane loses its negative charge (becomes less negative), these gates open to allow sodium ions to flow in through the sodium channels. The sodium channel valve is the main factor of action potential in the nerves.
• Chemistry portals. Some protein channel gates open by attaching another molecule to the protein. This causes a structural change in the protein molecule that opens or closes the gate. This valve is called a chemical valve (or ligand). One of the most important examples of chemical control is the effect of acetylcholine on the neuromuscular "acetylcholine channel" junction.
Facilitated diffusion is also called carrier-mediated diffusion. The substance thus transported cannot cross the cell membrane without the help of a specific carrier protein. The promoted dissemination consists of two stages:
(1) The transported molecule enters the blind end channel and binds to a specific receptor.
(2) Due to structural changes in carrier proteins, channels open on the opposite side of the membrane.
Facilitated dissemination differs from simple dissemination in the following important points: The simple diffusion rate increases in proportion to the concentration of the diffusing material. Facilitated diffusion maximizes the diffusion rate as the concentration of the substance increases. This maximum rate is determined by the rate at which a carrier protein molecule can undergo a conformational change.
• One of the most important substances that cross the cell membrane through easy diffusion is glucose and most amino acids.
Factors affecting net prevalence
Substances can diffuse through the cell membrane in both directions. Therefore, the net diffusion rate of a substance in the desired direction is usually important. This net rate is determined by the following factors:
• transmittance. Membrane permeability to a particular substance is expressed as the net diffusion rate of the substance per unit area of the membrane with respect to the difference in unit concentration on both sides of the membrane (unless there is an electrical or compressive difference). ..
• Difference in focus. The net rate of diffusion across the cell membrane is proportional to the difference in diffusion concentration across the membrane.
• Electric potential. When an electric potential is applied to the membrane, the ions move across the membrane due to the charge. When a large number of ions move across the transmembrane membrane, the difference in concentration of the same ion occurs in the opposite direction of the difference in potential. When the concentration difference rises to a sufficient level, the two effects balance each other and create an electrochemical equilibrium. The electrical difference that balances a given concentration difference can be determined using the Nernst equation.