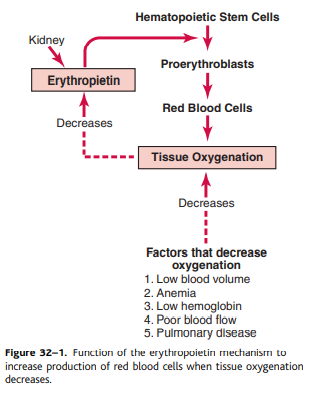
The total amount of red blood cells in the circulatory system is regulated in a narrow range. Any condition that reduces the amount of oxygen carried by the tissue usually increases the rate at which red blood cells are produced. The main factor that stimulates the production of red blood cells is the circulatory hormone erythropoietin.
In normal humans, about 90% of the red blood cell lopoietin is made in the kidneys and most of the rest is made in the liver. The structure of the kidney where erythropoietin is formed is unknown. Some studies have shown that erythropoietin is secreted by intercellular fibroblast cells, such as corticic and external medullary tubes that occur many consumption of oxygen cortices and renal. Other cells, including renal epithelial cells, secrete erythropic secretion in response to hypoxia (Fig. 32-1).
When the two kidneys are removed or prevented by renal disease, the amount of erythropoietin in non-cerebral tissues is sufficient to form a red blood cell in a red red cell so that the person is very low. Body
Vitamin B 12 and folic acid are important for the final maturity of red blood cells. Both vitamin B12 and folic acid are essential for DNA synthesis. A deficiency of any of these vitamins reduces the amount of DNA, resulting in failure of nuclear maturation and division. Except for non-proliferation, red blood cells grow larger than normal and develop into megablasts. These cells are irregular in shape and have a thin cell membrane. They can usually carry oxygen, but their fragility makes them short-lived. 1/2 to 1/3 of normal life. Therefore, a deficiency of vitamin B12 or folic acid causes impaired maturation during the process of red blood cell production. The common cause of mature maturity is Inlabil to absorb vitamin B12 in the digestive system. This is often occurring in staff with malignant anemia, a disease with mucosa for muscle gas base. The cells within the above cells are integrated with 12 vitamin B to secrete sugar proteins which are called one factor for vitamin B12. It is associated with strong unique factors with vitamin B12 and protecting vitamins from digestive enzymes. TOR-VITAMIN B12 complex for the essential vitamin Tor novel with a reception location in the membrane membrane membrane membrane of the president. Vitamin B12 is brought by the Pinocytosis process. The lack of internal factor causes the most disadvantages of vitamins due to the enzyme during intestinal and absorption.
Formation of Hemoglobin
Synthesis of hemoglobin begins when red blood cells begin in the building and continues at the reticulocyte stage, at the point where the brain leaves the brain and goes to the bloodstream. During the formation of hemoglobin, he said the bean COM molecule with a long polypeptide series to create a glain called sububaite hemoglobin. Four chains of hemoglobin are loose to form the entire hemoglobin molecule. The most important feature of hemoglobin moles is their ability to bind freely and reversibly to oxygen. The oxygen atom is loosely linked to one of the so-called coordination bonds of the iron atom in hemoglobin. When bound to heme iron, oxygen is transported as molecular oxygen, which consists of two oxygen atoms. Oxygen is released in the form of molecular oxygen dissolved in tissue fluids instead of ionic oxygen.
Iron Metabolism
Iron is important for the formation of hemoglobin, myoglobin and other substances such as cytochrome, cytochrome oxidase, peroxidase and catalase. The total amount of a total iron in the body is about 4 to 5 grams. About 65% of this value takes place in the form of hemoglobin. Approximately 4% is in myoglobin form, 1% are in the form of different hem compounds that promote intracellular oxidation, 0.1% with protein protein in blood plasma and 15 to 30% are mainly stored. Retikuliergothel system and the physical cells of the liver, mainly in the form of ferritin
Iron is shipped and stored. When iron is absorbed by the small intestine, container immediately converts with a globulin B called apotrantrin, which is worn in plasma. This iron is slightly limited. Excess iron in the blood is deposited in hepatocytes and retinal endothelial cells of the bone marrow. Once the iron enters the cell's cytoplasm, it combines with the protein apoferitin to form ferritin.
Different amounts of iron can combine in ferritin to form groups of iron radicals. When the amount of iron in the plasma falls below normal, the iron can be easily removed from the ferritin and carried by transferrin in the plasma to the parts of the body that need it. The unique feature of the transferrin molecule is its ability to bind strongly to receptors in the membranes of erythroblasts and bone marrow. Transferrin is introduced into the erythroblast with iron bound by endocytosis. Transferrin transports iron directly to the mitochondrial cartilage, where heme is synthesized.
When the red blood cells reach the end of their life and are destroyed, the hemoglobin that was released is taken up by the cells of the monocyte macrophage system. Free iron can be stored in the ferritin pool or reused to form hemoglobin.
Book Refrence: Pocket Guyton(twelth edition)